Titanium is a master of disguise. You see it building the skeletal frames of supersonic jets, yet you also unknowingly eat it as a white powder additive in donuts. You are told it is one of the strongest materials on Earth, yet if you pick up a titanium ring, it feels unnervingly light—almost like plastic.
These contradictions lead to a persistent confusion: Is titanium actually a metal?
The short answer is yes. Titanium (symbol: Ti) is a transition metal located in Group 4 of the periodic table. It possesses all the classic hallmarks of a metal: it is lustrous, silver in color, conducts electricity, and is malleable. However, unlike steel or aluminum, titanium occupies a unique middle ground in physics and chemistry that often tricks our senses.
This guide goes beyond the simple “yes or no.” Drawing from metallurgical standards (ASTM) and practical workshop experience, we will unpack the atomic structure of titanium, explain why 95% of the world’s titanium isn’t used as metal at all, and reveal why this “man-made” extraction feels so different in your hand.
The Chemistry: Is Titanium a Metal or Non-Metal?
To understand the nature of titanium, we must look at where it lives chemically. If you consult a periodic table, you will find titanium at Atomic Number 22, nestled in the “d-block.”
Where Ti Fits in the Periodic Table
Titanium is scientifically classified as a transition metal. In chemistry, this means it has partially filled d-orbitals of electrons. This specific electron configuration is what gives titanium its legendary strength-to-weight ratio and its ability to form stable alloys.
If you were to isolate pure titanium in a vacuum, it would verify its metallic status immediately:
- Luster: It has a shiny, metallic silver appearance.
- Conductivity: It conducts heat and electricity (though not as efficiently as copper).
- Malleability: It can be hammered or rolled into sheets without breaking.
According to the Royal Society of Chemistry, titanium also has a melting point of over 1,668°C (3,034°F), placing it firmly in the category of refractory metals alongside tungsten and molybdenum.
Why Some People Think It’s a Non-Metal (The TiO₂ Factor)
If the science is so clear, why do people search “Ti metal or nonmetal” thousands of times a month? The confusion often stems from how we encounter titanium in daily life.
In the natural world, you will almost never find a chunk of shiny titanium metal lying on the ground. Instead, titanium is a “lithophile” (rock-loving) element that bonds aggressively with oxygen. The result is Titanium Dioxide (TiO₂), a chalky, white powder.
This is the form used in:
- Paints and Coatings: To provide bright white opacity.
- Sunscreen: To block UV rays physically.
- Food Additives: To brighten candies and pastries.
Because the vast majority of global titanium production is refined into this white, non-metallic powder rather than solid metal ingots, it is easy to see why the line blurs for the average consumer. While the oxide behaves like a ceramic or mineral, the element itself is undeniably a metal.
What Is Titanium Made Of? (Atoms vs. Alloys)
When you ask, “What is titanium made of?” the answer depends on whether you are in a chemistry lab or a machine shop.
At the Atomic Level
Titanium is an element, meaning it is not made of other substances mixed together. It is made of titanium atoms—specifically, atoms with 22 protons in the nucleus. It is distinct from steel (an alloy of iron and carbon) or bronze (an alloy of copper and tin), which are mixtures.
Practical Guide: The Critical Difference Between Grade 1 and Grade 5
In the consumer market, “titanium” is a vague label. To avoid overpaying for soft metal or buying “aerospace” gear that is actually industrial pipe material, you must understand ASTM Grades.
1. Commercially Pure (CP) Titanium (Grades 1-4)
- Composition: >99% Titanium.
- Characteristics: Excellent corrosion resistance, but relatively soft and ductile. It bends easily compared to steel alloys.
- Real-World Use Case: Camping Mugs & Chemical Pipes. If you buy a titanium camping pot, it is likely Grade 1 or 2. Why? Because it needs to be pressed into shape (deep drawing) without cracking. It doesn’t need to hold an edge or withstand high impact.
2. Titanium Alloy (Grade 5 / Ti-6Al-4V)
- Composition: 90% titanium, 6% aluminum, 4% vanadium.
- Characteristics: The “Alpha-Beta” alloy. It has double the tensile strength of CP titanium. It is heat-treatable and extremely tough.
- Real-World Use Case: Pocket Knife Handles & Aerospace Bolts. If you are buying a titanium framelock knife or a high-end watch, you want Grade 5. CP titanium would be too soft, causing the lock mechanism to deform or “stick.”
Buyer’s Warning: Many cheaper “Titanium” keychains or EDC tools use Grade 2 because it is easier to machine (softer), but they sell it at Grade 5 prices. Always ask: “Is this Ti-6Al-4V or CP?”
Is Titanium Man-Made or Natural?
This is a semantic trap. Titanium is a natural element, but titanium metal is a man-made product.
Natural Abundance
Titanium is not rare. In fact, data from the U.S. Geological Survey (USGS) confirms it is the ninth most abundant element in the Earth’s crust. It is found in minerals like ilmenite and rutile, which look like black sand.
The Kroll Process: Why It Costs So Much
If it’s as common as dirt, why is it expensive? Because extracting the metal from the ore is a nightmare of chemical engineering.
Unlike gold, which can be panned from a river, titanium hates being pure. To separate it from oxygen, engineers use the Kroll process. This involves:
- Heating the ore with chlorine gas to create liquid titanium tetrachloride (TiCl₄).
- Reacting that liquid with molten magnesium in a vacuum at extreme temperatures.
- Crushing the resulting “titanium sponge” and melting it down again.
This process consumes massive amounts of energy and takes days. So, while the atoms are natural, the shiny metal bar is the result of intense human industrial effort.
The “Fake” Test: How to Verify Titanium
One of the most jarring experiences for new buyers is the “plastic feel.” You pick up a supposedly expensive metal watch, but it doesn’t have the reassuring heft or the icy cold touch of steel. Is it a fake?
Here are three field tests used by machinists and collectors to verify titanium:
1. The Density Factor (The Heft Test)
Titanium has a density of roughly 4.5 g/cm³, whereas steel is around 7.8 g/cm³. This means for the same volume, titanium is nearly 45% lighter than steel. Our brains often associate “quality metal” with “heavy,” so the lightness of titanium creates a cognitive dissonance, mimicking the weight of dense plastics.
2. The Thermal Conductivity Paradox (The Touch Test)
Touch a piece of aluminum or steel; it feels cold. That is because it rapidly sucks heat away from your fingertips. Titanium, however, is a poor conductor of heat.
- Aluminum: ≈ 205 W/(m · K)
- Titanium: ≈ 17 W/(m · K)
Because titanium conducts heat slowly, it doesn’t sap your body heat instantly. It feels “warm” or neutral to the touch—much like a polymer would. This low thermal conductivity is actually a sign of authenticity, not a defect.
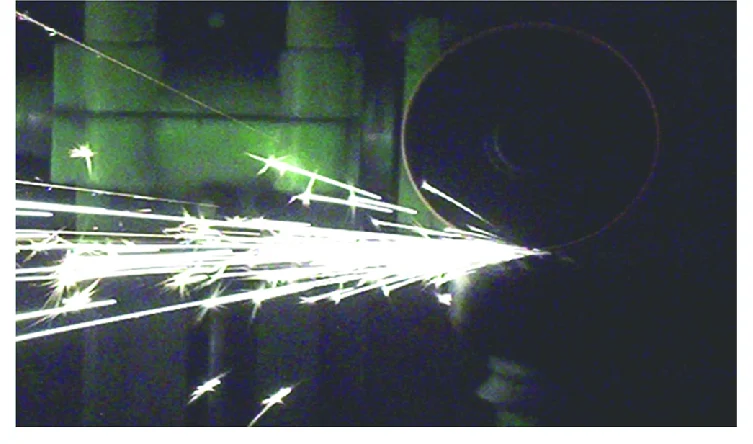
3. The Spark Test (The Shop Test)
Warning: Do not attempt this on finished jewelry. If you touch a piece of raw titanium to a grinding wheel, it produces a shower of brilliant, blinding white sparks.
- Steel sparks: Yellow/orange and forked.
- Titanium sparks: Pure white and incredibly bright (like a miniature firework). This is the definitive way recyclers distinguish titanium from stainless steel.
Is Titanium Toxic? The “Heavy Metal” Myth
The term “heavy metal” is often used loosely to describe toxic environmental hazards like lead or mercury. Is titanium one of them?
Physically, titanium is a “light metal” due to its low density. Toxicologically, it is considered biocompatible and inert.
This safety profile is why titanium is the gold standard for medical implants. It goes beyond simply being “non-toxic.” Titanium exhibits a phenomenon called osseointegration. According to research available through the National Center for Biotechnology Information (NCBI), bone tissue can actually grow into the microscopic rough surface of titanium, structurally fusing the metal with the human skeleton.
Comparison: Titanium vs. Other Metals
To help you decide if titanium is the right material for your needs, here is a quick breakdown against its main rivals.
| Feature | Titanium (Grade 5) | Stainless Steel (316L) | Aluminum (6061) |
|---|---|---|---|
| Weight | Light | Heavy (2x Titanium) | Very Light (60% of Titanium) |
| Strength | Very High | High | Moderate |
| Rustproof? | Yes (Immune to saltwater) | Mostly (Can pit) | Oxidizes/Pits over time |
| Feel | Warm, Lightweight | Cold, Heavy | Neutral, Featherweight |
| Cost | $$$ | $ | $ |
| Common Issue | Galling (Threads stick) | Heavy | Fatigue over time |
Pro Tip on Galling: Titanium threads are notorious for “cold welding” or galling. If you have a titanium bolt or flashlight cap, always use a lubricant. If it gets stuck, do not force it; you might fuse the metal permanently.
Frequently Asked Questions
Is titanium a metal or non-metal?
Titanium is a transition metal. The confusion arises because its most common commercial form is titanium dioxide, a white powder used as a pigment.
Is titanium magnetic?
No, titanium is paramagnetic. It interacts very weakly with magnetic fields, making it safe for MRI scans (though you should always consult your doctor).
Does titanium rust?
Titanium does not rust in the traditional sense. It forms a stable, protective oxide layer immediately upon exposure to air, which prevents corrosion even in saltwater.
Is titanium stronger than diamond?
No. Diamond is harder (resistant to scratching), but titanium has higher tensile strength (resistant to breaking or stretching). You can scratch titanium, but you can’t shatter it like a diamond.
Is titanium man-made?
Titanium atoms are natural and abundant. However, pure titanium metal does not exist naturally on Earth and must be extracted using the man-made Kroll process.
Source Transparency & References
This article prioritizes data from standard-setting bodies and scientific institutions. Key references include:
- ASTM B265 & B348: Standard Specifications for Titanium and Titanium Alloy Strip, Sheet, and Plate.
- USGS Mineral Commodity Summaries: For abundance and production data.
- Royal Society of Chemistry: For atomic properties.
- NCBI/NIH: For biocompatibility studies.