The short answer is no, titanium does not rust.
In scientific terms, “rust” is a specific chemical reaction reserved for iron and its alloys (like steel) when they are exposed to moisture and oxygen, creating iron oxide (Fe2O3). Because titanium is a non-ferrous metal—meaning it contains no iron—it is chemically impossible for it to rust.
However, while titanium does not rust, it is not immune to all forms of corrosion. It is highly resistant, but under specific extreme conditions defined by chemical engineering limits, it can degrade.
The Science: The 10-Nanometer Shield
Titanium’s corrosion resistance is not magic; it is quantifiable chemistry known as passivation.
When raw titanium is exposed to oxygen, it instantly forms a microscopically thin layer of Titanium Dioxide (TiO2) on its surface.
- Formation Speed: This reaction occurs in nanoseconds.
- Thickness: The layer is initially only 1-2 nanometers thick but can thicken to 5-10 nanometers over time (for context, a human hair is roughly 80,000 nm thick).
- Stability: Despite its thinness, this film is chemically stable within a pH range of 2 to 12.
- Standard Performance: In standardized Salt Spray Tests (ASTM B117), Titanium Grade 2 and Grade 5 typically show zero corrosion rates after thousands of hours of exposure, whereas 316L stainless steel often shows signs of pitting after 1,000 hours under similar conditions.
Engineering Boundaries: When Titanium Fails
Titanium is not invincible. For engineers and industrial users, strictly observing the following operational boundaries is critical:
1. Dry Chlorine Gas (The Water Limit)
Titanium is immune to wet chlorine but can ignite in dry chlorine due to a rapid exothermic reaction.
- The Boundary: To maintain the protective passivation layer, the water content in chlorine gas must be greater than 0.5% (industry standards often recommend >1.5% for safety margin). Below this moisture threshold, the TiO2 layer cannot regenerate.
2. Reducing Acids (Concentration Limits)
Titanium resists oxidizing acids (like Nitric Acid) well, but fails in reducing acids without corrosion inhibitors:
- Hydrochloric Acid (HCl): Unalloyed titanium (Grade 2) is generally resistant only up to 5% concentration at room temperature. Above 10% concentration or temperatures exceeding 60°C (140°F), the corrosion rate increases significantly.
- Sulfuric Acid (H2SO4): Similarly, titanium is stable only at very low concentrations (<5%) or low temperatures.
3. Galvanic Corrosion
If titanium is coupled with a dissimilar metal (like aluminum or carbon steel) in an electrolyte (like saltwater), titanium acts as the noble metal (cathode). It will not corrode, but it will accelerate the corrosion of the anode (the other metal).
Performance in Saltwater vs. Stainless Steel (The PREN Factor)
To scientifically compare rust resistance, engineers use the Pitting Resistance Equivalent Number (PREN). A higher number indicates greater resistance to pitting corrosion in saltwater.
PREN Formula: PREN = %Cr + 3.3 x %Mo + 16 x %N
| Material | Typical PREN Score | Corrosion Risk in Seawater |
|---|---|---|
| 304 Stainless Steel | ~18 – 20 | High (Will rust over time) |
| 316L Stainless Steel | ~24 – 26 | Moderate (Risk of pitting/crevice corrosion) |
| Super Duplex Steel | > 40 | Very Low |
| Titanium (Grade 2/5) | N/A (Immune) | Zero Risk |
Note: While PREN is designed for steel, titanium behaves as if it has a PREN score well above 40. In heavily chlorinated water (swimming pools), data shows titanium exhibits a corrosion rate of < 0.001 mm/year, effectively rendering it permanent.
Why Titanium May Discolor
Users sometimes mistake surface discoloration for rust. If a titanium item looks dull or dark, it is usually due to one of the following reasons:
- Surface Accumulation: Titanium easily collects oils, fingerprints, and soap scum. This buildup can appear brownish or dark.
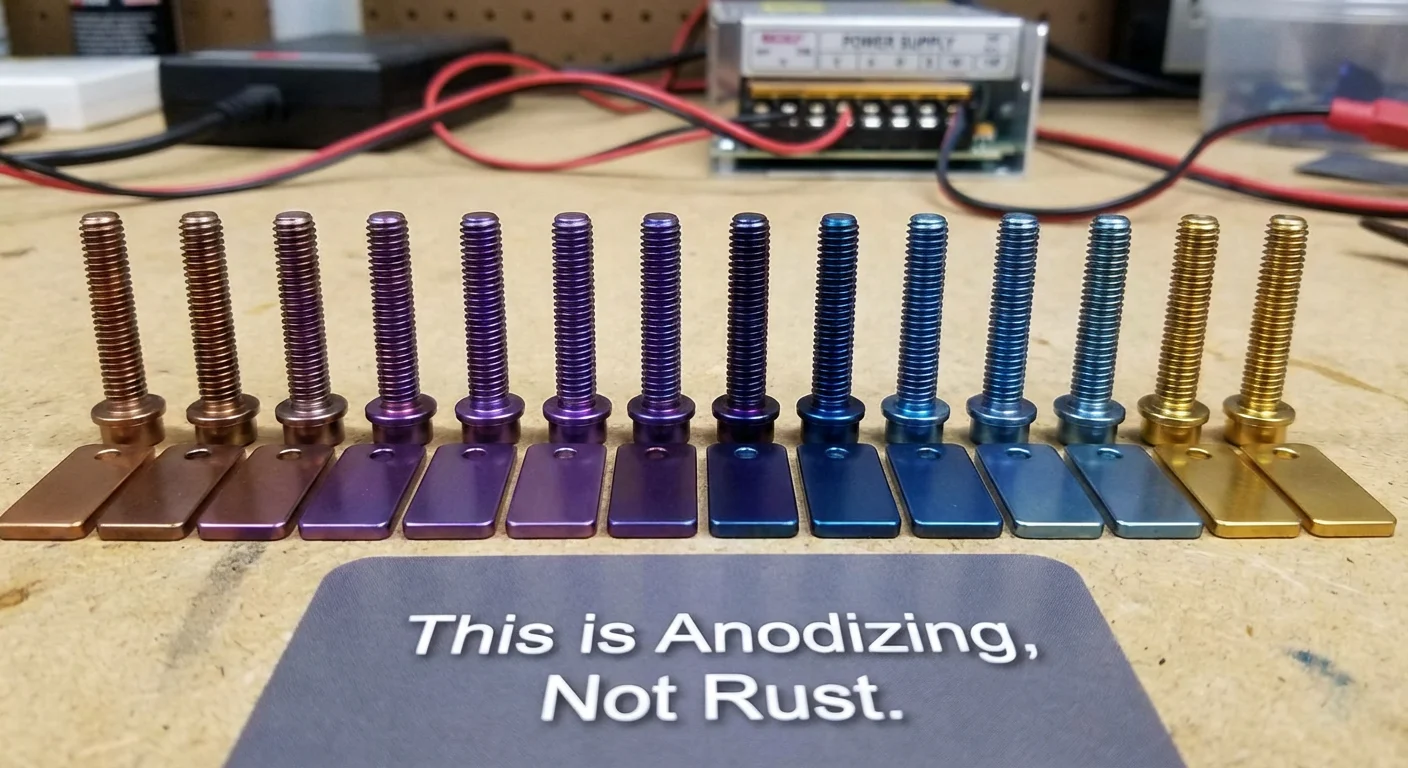
- Anodizing: Exposure to high heat (>300°C) or electrical voltage can thicken the oxide layer, changing light refraction and causing the metal to appear blue, bronze, or purple. This is a surface phenomenon, not structural damage.
How to Clean Titanium:
- Wash with warm water and mild dish soap.
- Scrub gently with a soft toothbrush to remove debris.
- Rinse and dry with a microfiber cloth.
Conclusion
Titanium does not rust because it lacks iron. Its natural ability to form a self-healing titanium dioxide layer makes it virtually immune to corrosion in saltwater, swimming pools, and standard atmospheric conditions.
While it has specific metallurgical limitations in dry chlorine and hot reducing acids, for most applications—including marine engineering, medical implants, and consumer goods—titanium offers superior durability that exceeds stainless steel standards.
Common Questions
Q: Will titanium turn skin green?
No. Green skin is typically caused by a reaction between skin acidity and copper or nickel. Titanium is biocompatible and chemically inert, meaning it does not react with skin oils or sweat.
Q: Does titanium scratch?
Yes. While resistant to corrosion, titanium is not scratch-proof. Over time, it may develop fine surface scratches, forming a matte finish (patina). The oxide layer heals over these scratches instantly, maintaining rust protection.
Q: Is titanium magnetic?
No. Titanium is paramagnetic and effectively non-magnetic. This property is often used to distinguish it from steel alloys in medical environments (MRI safe).
Q: How can I tell if it is real titanium?
Real titanium is ~45% lighter than steel. In a workshop setting, a grind test is definitive: grinding titanium produces brilliant white sparks, whereas steel produces yellow/orange sparks.